Virtual Molecular Tumor Board
The VICC is currently leading a virtual molecular tumor board. The purpose of this project is to drive discussions on how to develop new tools, guidelines, standards, ontologies, APIs, best practices, etc. that facilitate clinical interpretation of cancer variants. The idea of using a virtual tumor board was inspired in part by the physical tumor boards of participating institutions as well as the ASCO University Molecular Oncology Tumor Board.
VICC VMTB currently runs two different series and one study regarding NGS Reporting Practices (see below for more information).
ClinGen Somatic Cancer and VICC Virtual Molecular Tumor Board Case Series
The CME Case Series is a monthly series, which is accredited for medical education with ACMG (https://www.acmgeducation.net). Each presentation in the case series features oncologists and clinical laboratory scientists as speakers. The oncologist usually presents the clinical case vignette and the clinical laboratory scientist will present the genomic data and interpretation.
Variant Type Series
The Variant Type Series aims to orient and introduce the members of the VMTB to variants that are outside of the typical SNV/indels that are well documented in the literature and data/knowledgebases. In the calls, the presenters describe the assays that are capable of detecting these variants and if feasible, point out good resources for interpreting clinical data and gaps in knowledgebase/database representation. The Variant Type Series is an open format with lively discussions, which features topics such as
- alternative splicing,
- applications for whole transcriptome RNAseq,
- the use of cf/ct DNA to detect biomarkers.
If you would like to participate in the Virtual Tumor Board by submitting a hypothetical case or discussing cases with the group, please contact the Project Leaders below.
An international Landscape of Cancer NGS Reporting Practices
Multiple guidelines and standards have been issued globally to aid in Next Generation Sequencing (NGS) variant interpretation. The AMP/ASCO/CAP, ACMG/AMP, and ESMO published guidelines are incorporated into cancer NGS reports in different ways around the globe. The Variant Interpretation for Cancer Consortium Virtual Molecular Tumor Board (VICC-VMTB), in collaboration with the Cancer Genomics Consortium (CGC) and the ClinGen Somatic Clinical Domain Working Group (CDWG), distributed a comprehensive survey to members membership of each group regarding reporting practices for cancer NGS testing worldwide with the goal to
- come to a consensus on essential and/or preferred data elements in a cancer genomics NGS report, and
- provide guidance on reporting cancer genomic testing results for use by a molecular tumor board.
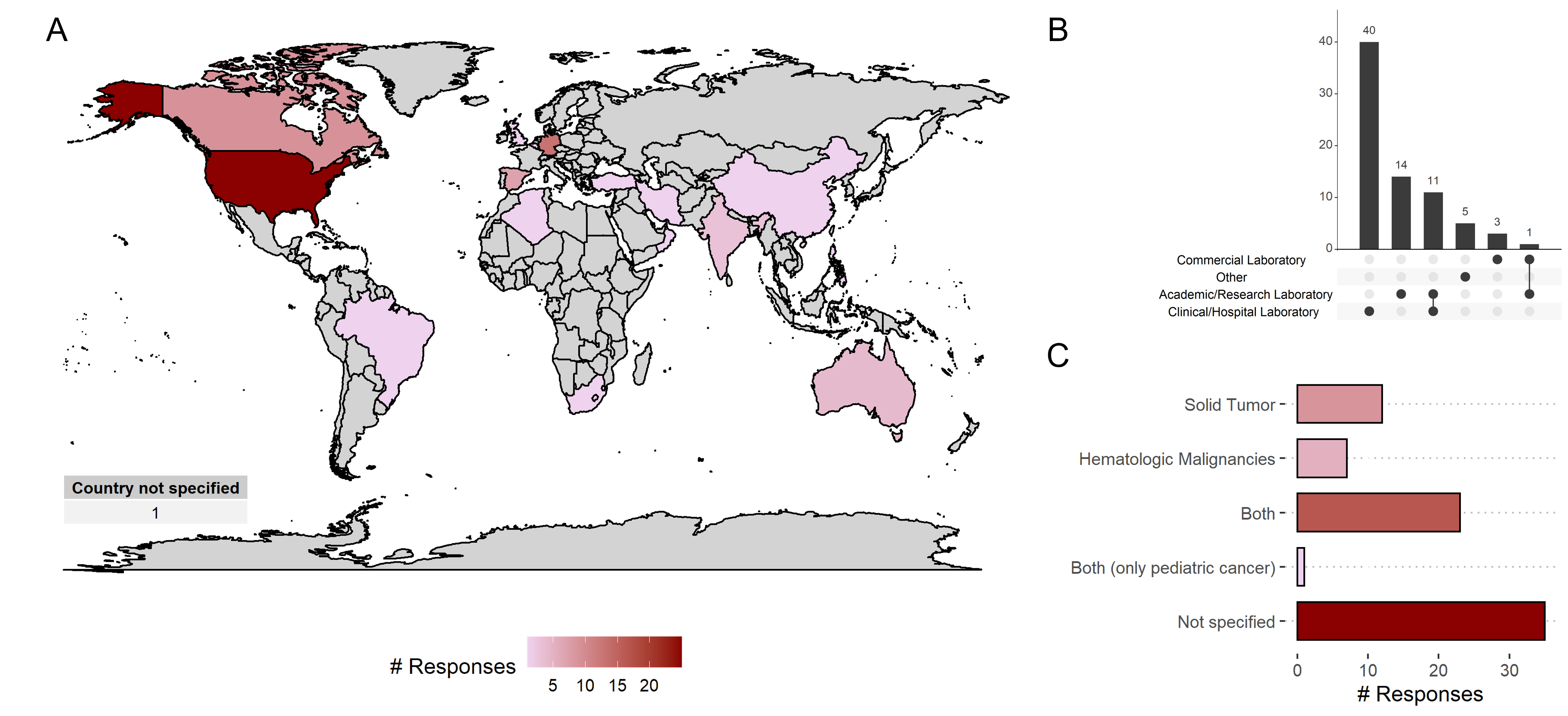 Figure 1: A) Number of participants from different countries. B) Background of participants. C) Type of tumors discussed in NGS reporting.
Figure 1: A) Number of participants from different countries. B) Background of participants. C) Type of tumors discussed in NGS reporting.
The use of reporting elements both consistently and differentially is currently evaluated and the survey is open until September 30, 2022
Publications
Project Leaders
- Beth Pitel, MS, CG(ASCP)CMMayo Clinic
- Jason Saliba, PhDWashington University School of Medicine in St. Louis
- Manuela Benary, MSc, PhDCharité - Universitätsmedizin Berlin


