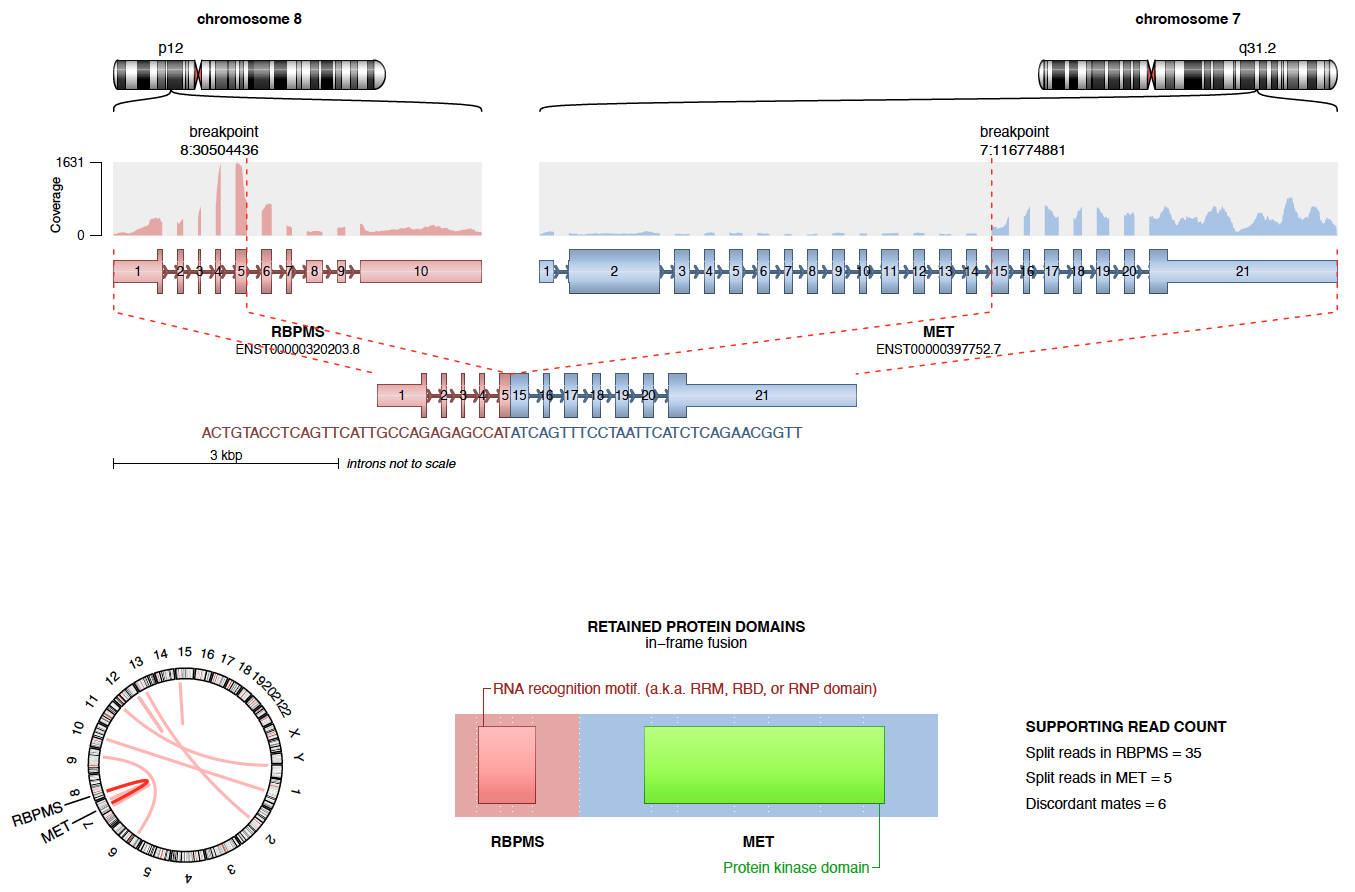
Gene Fusion Oncogenicity
Objectives and Scope
The recently developed ClinGen/CGC/VICC SOP for the classification of oncogenicity of somatic variants in cancer defined the classification of gene fusions as out of scope, due to nuances specific to such alterations. Similarly, the cross-consortia (ClinGen, CGC, VICC) gene fusion characterization project (fusions.cancervariants.org) is specifying the minimal information for gene fusions as a class of variation but intentionally excludes guidance on the clinical classifications of those variants.
We seek to complement these efforts by developing systematic recommendations and procedures for classification of gene fusion pathogenicity (oncogenicity) in cancer. Examples of criteria for classification of oncogenicity might include, but are not limited to, function of partner genes, 5’-3’ orientation, inclusion of key domains, maintenance of the reading frame, association of the fusion itself or partner genes with cancer, absence in normal tissues, functional studies, etc.
Alignment of this project with related projects, such as the gene fusion representation project, the standards for classification of somatic variants in cancer (PMID: 36063163) and the ClinGen NTRK fusion oncogenicity guidelines will be accomplished by including members in the working group who were involved in these other initiatives. The community outreach and engagement plan (see Community Outreach and Engagement) should also help to ensure alignment with these other efforts.
Project Participants
This project will include two leads, an advisory committee, a working group, and a project coordinator.
Project Leaders
- Catherine Cottrell, PhD, FACMGNationwide Children's Hospital, The Ohio State University
- Karen Tsuchiya, MD, FCAP, FACMGNationwide Children's Hospital, The Ohio State University
Project Members
- Albrecht Stenzinger, MDUniversity Hospital Heidelberg
- Angshumoy Roy, MBBS, PhDBaylor College of Medicine
- Ying-Chen Claire Hou, PhDNationwide Children's Hospital, The Ohio State University
- Deborah White, PhD, FFSc (RCPA)South Australian Health & Medical Research Institute
- Julia Bridge, MDProPath & University of Nebraska Medical Center
- Paulo Campregher, MD, PhDHospital Israelita Albert Einstein
- Valentina Nardi, MDHarvard Medical School
- Vera Paulson, MD, PhDUniversity of Washington
Advisory Committee
- Alex H. Wagner, PhDNationwide Children's Hospital, The Ohio State University
- Gordana Raca, MD, PhD, FACMGChildren's Hospital Los Angeles, Keck School of Medicine of USC
- Yassmine Akkari, PhD, FACMGNationwide Children's Hospital, The Ohio State University
Project Coordinator
Contact Information
Please contact Wesley Goar for more information on this project.













